Title
Chapter 2.1: Visualization of Metal Ion Buffering Via 3-D Topo Surfaces of Complexometric Titrations
Files
Download Chapter (1.4 MB)
Description
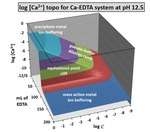
This chapter examines 1:1 metal-ligand complexometric titrations in aqueous media. It presents surfaces that plot computed equilibrium parameters above a composition grid with titration progress (mL of ligand) as the x-axis and overall system dilution (log C ) as the y-axis. The sample systems in this chapter are restricted to EDTA as a ligand. Other chelating ligands that form exclusively 1:1 complexes could also be modeled with this software. The surfaces show the quality of the equivalence point break under various conditions. More importantly, they develop the phenomenon of metal ion buffering. They clearly distinguish the difference between “pseudo-buffering” and “true buffering”. They introduce terminology for two different forms of metal ion buffering: 1) mass action metal ion buffering under excess ligand conditions; and 2) precipitate metal ion buffering when hydroxide precipitates are present under excess metal ion conditions. Systems modeled are EDTA titrations of Cu2+, Ca2+ and Mg2+. A final section demonstrates a second type of topo that helps evaluate the optimal pH for an EDTA titration. Supplemental files include the Complexation TOPOS software, an Excel workbook that generates topo surfaces in under 20 seconds, and teaching suggestions. Required inputs are: 1) stability constants for the metal-ligand complex; 2) acid dissociation constants for the ligand, 3) stability constants for hydroxy complexes from of the metal cation; and 4) a Ksp value and stoichiometry for hydroxide precipitates. Many of these constants are contained in a workbook tab. Also included are a PowerPoint lecture and teaching materials (for lecture, homework, and pre-laboratory activities) that are suitable in general chemistry courses or third-year and graduate courses in analytical chemistry, biochemistry and geochemistry.
Publication Date
4-2021
Document Type
Book
Rights
© 2021 Garon C. Smith and Md Mainul Hossain
Creative Commons License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
Disciplines
Chemistry
Recommended Citation
Smith, Garon C. and Hossain, Md Mainul, "Chapter 2.1: Visualization of Metal Ion Buffering Via 3-D Topo Surfaces of Complexometric Titrations" (2021). Water Topos: A 3-D Trend Surface Approach to Viewing and Teaching Aqueous Equilibrium Chemistry. 5.
https://scholarworks.umt.edu/topos/5
Comments
This document is the Accepted Manuscript version of a Published Work that appeared in final form in the Journal of Chemical Education, copyright American Chemical Society and the Division of Chemical Education, Inc., after peer review and technical editing by the publisher. Access the final edited and published work.