Files
Download Chapter (1.4 MB)
Download Chapter 2.2: Anti-Buffering TOPOS software (8.5 MB)
Download Chapter 2.2: Anti-Buffering TOPOS PowerPoint lecture (5.7 MB)
Download Chapter 2.2: Teaching materials (1.7 MB)
Download Chapter 2.2: Anti-Buffering Confirmation (2.0 MB)
Download Chapter 2.2: Derivation of equations (172 KB)
Download Chapter 2.2: Documented code listing (30 KB)
Description
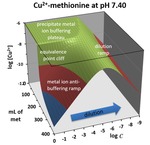
Diluting a system that contains metal complexes can sometimes cause surprises. This chapter describes “metal ion anti-buffering”, a situation in which free metal ion concentrations rapidly increase as system dilution drives dissociation. It only occurs under excess free ligand conditions when a solution is dominated by higher stoichiometry complexes. The Law of Mass Action is used to provide a mathematical justification for the phenomenon. A Cu2+-ethylenediamine mixture exhibits this phenomenon when excess free ethylenediamine (en) is present. For example, it occurs when diluting a solution containing a four-fold excess of en over Cu2+. As this mixture is diluted by a factor of ~5600, the modeled free Cu2+ concentration shows a ~470-fold increase. Taken together, this is 2.5 million times higher than dilution of the system would yield in other circumstances. Included are experimental data confirming anti-buffering in the Cu2+-en system. Many other metal-ligand systems can display this behavior. Four additional examples are illustrated including an amino acid under physiological pHs. Anti-Buffering TOPOS, a downloadable Excel workbook in a supplemental file, allows readers to simulate this behavior for many metal-ligand systems. A PowerPoint lecture and teaching materials are also provided, suitable for inclusion in upper division and graduate courses in analytical chemistry, biochemistry and geochemistry.
Publication Date
7-2021
Document Type
Book
Rights
© 2021 Garon C. Smith, Md Mainul Hossain, Daniel D. Barry
Creative Commons License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
Disciplines
Chemistry
Recommended Citation
Smith, Garon C.; Hossain, Md Mainul; and Barry, Daniel D., "Chapter 2.2: 3-D Topo Surface Visualization of Metal Ion Anti-Buffering: An Unexpected Behavior in Metal–Ligand Complexation Systems" (2021). Water Topos: A 3-D Trend Surface Approach to Viewing and Teaching Aqueous Equilibrium Chemistry. 6.
https://scholarworks.umt.edu/topos/6
Comments
This document is the Accepted Manuscript version of a Published Work that appeared in final form in the Journal of Chemical Education, copyright American Chemical Society and the Division of Chemical Education, Inc., after peer review and technical editing by the publisher. Access the final edited and published work.