By Garon C. Smith, Department of Chemistry and Biochemistry at University of Montana, and Md Mainul Hossain, Department of Biochemistry and Microbiology at North South University, Dhaka, Bangladesh
This book outlines a new approach to viewing and teaching aqueous equilibrium chemistry, a method that employs computer-generated trend surfaces (topos) to broadly summarize chemical process that can occur in water solutions. Topics include acid-base reactions, metal-ligand complexation, oxidation-reduction processes and solubility. Each chapter provides a concept paper plus additional resources as supplementary files: free downloadable software to generate your own 3-D trend surfaces, PowerPoint tutorial/lecture slides, a “Teaching with…” document, extended background topics, equation derivations, etc.
IMPORTANT NOTE: The free downloadable software for each chapter is found in an Excel workbook that contains executable code. Because of this, they will probably trigger security warnings that prevent the software from operating. We have found that if you copy the downloaded Excel workbook to a directory on your computer, this will eliminate the security risk blockage. More complete instructions for this procedure are found in the downloaded software for each chapter.
-
Prologue: An Overview to Water Topos: A 3-D Trend Surface Approach to Viewing and Teaching Aqueous Equilibrium Chemistry
Garon C. Smith and Md Mainul Hossain
The “topo” approach to viewing and teaching aqueous equilibrium through 3-D trend surfaces is introduced. A list of the pedagogical resource categories that accompany each chapter is provided. We describe the “composition grids” that form the foundation for the trend surfaces. A roadmap for the ... Read More
-
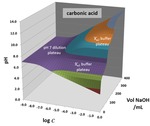
Chapter 1.1: 3-D Surface Visualization of pH Titration “Topos”: Equivalence Point Cliffs, Dilution Ramps and Buffer Plateaus
Garon C. Smith, Md Mainul Hossain, and Patrick MacCarthy
3-D topos can be generated to visualize how pH behaves during titration and dilution procedures. The surfaces are constructed by plotting computed pH values above a composition grid with volume of base added in one direction and overall system dilution on the other. Surficial features ... Read More
-
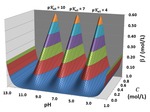
Chapter 1.2: Visualization of Buffer Capacity with 3-D Topos: Buffer Ridges, Equivalence Point Canyons and Dilution Ramps
Garon C. Smith and Md Mainul Hossain
The BufCap TOPOS software generates 3-D topographic surfaces for acid-base equilibrium studies that portray pH and buffer capacity behavior during titration and dilution procedures. Topo surfaces are created by plotting computed pH and buffer capacity values above a composition grid with volume of NaOH as ... Read More
-

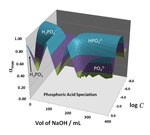
Chapter 1.3: 3-D Topo Surface Visualization of Acid-Base Species Distributions: Corner Buttes, Corner Pits, Curving Ridge Crests and Dilution Plains
Garon C. Smith and Md Mainul Hossain
This chapter adds 3-D species distribution topos to earlier surfaces that showed pH (Chapter 1.1) and buffer capacity behavior (Chapter 1.2) during titration and dilution procedures. It constructs trend surfaces by plotting computed alpha distribution coefficients above a composition grid with “mL of NaOH” as ... Read More
-
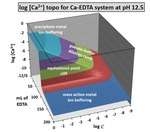
Chapter 2.1: Visualization of Metal Ion Buffering Via 3-D Topo Surfaces of Complexometric Titrations
Garon C. Smith and Md Mainul Hossain
This chapter examines 1:1 metal-ligand complexometric titrations in aqueous media. It presents surfaces that plot computed equilibrium parameters above a composition grid with titration progress (mL of ligand) as the x-axis and overall system dilution (log C ) as the y-axis. The sample systems in ... Read More
-
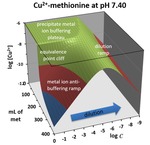
Chapter 2.2: 3-D Topo Surface Visualization of Metal Ion Anti-Buffering: An Unexpected Behavior in Metal–Ligand Complexation Systems
Garon C. Smith, Md Mainul Hossain, and Daniel D. Barry
Diluting a system that contains metal complexes can sometimes cause surprises. This chapter describes “metal ion anti-buffering”, a situation in which free metal ion concentrations rapidly increase as system dilution drives dissociation. It only occurs under excess free ligand conditions when a solution is dominated ... Read More
-
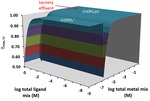
Chapter 2.3: Natural Attenuation of Chromium and Manganese from a Bangladesh Tannery Effluent Via Humic Substance Complexation: Field, Laboratory and Modeling Studies
Ayesha Sharmin, Md Mainul Huda, Mahabub Islam, Md Mainul Hossain, Garon C. Smith, Sohidul Islam, Mohammad Moshiur Rahman, Mohammad Hossain Shahriare, Mohsin Kazi, and Mohammad Jakariya
This chapter provides an example of the 3-D TOPOS visualization approach to a real-world application of metal-ligand complexation. It utilizes the Multi TOPOS software, an extension of Complexation TOPOS to include multi-metal/multi-ligand mixtures. It examines the impact of toxic chromium and manganese in effluents from ... Read More
-
Chapter 3.1: Visualization of the Nernst Equation Via 3-D Topo Surfaces: E⁰ Plateaus, Left-Hand Bluffs, Front Cliffs and Reaction Paths
Garon C. Smith and Md Mainul Hossain
A new 3-D graphical representation of oxidation–reduction (redox) processes in aqueous solutions has been developed utilizing a composition grid for which the x-axis carries the activity of the reduced form of the redox couple and the y-axis carries the activity of the oxidized form. The ... Read More
-
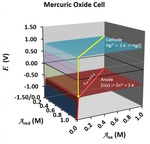
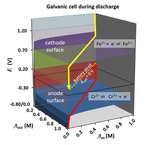
Chapter 3.2: Why Batteries Deliver a Fairly Constant Voltage Until They Suddenly Die: An Application of Nernst Topo Surfaces
Garon C. Smith, Md Mainul Hossain, and Patrick MacCarthy
Two characteristics of batteries, their delivery of nearly constant voltage and their rapid failure, are explained through a visual examination of the Nernst equation. Two Galvanic cells are described in detail: (1) a wet cell involving iron and copper salts and (2) a mercury oxide ... Read More
-
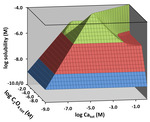
Chapter 4.1: Visualizing the Solubility of Salts Via 3-D Topo Surfaces: Pyramids with Ridges and Plateaus
Garon C. Smith and Md Mainul Hossain
A new 3-D graphical representation for the solubility of sparingly soluble salts in aqueous solutions has been developed utilizing a new composition grid. In this case, the x-axis carries the concentration of the salt’s cation (usually a metal) and the y-axis holds the concentration of ... Read More