Files
Download Chapter (1.3 MB)
Description
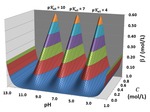
The BufCap TOPOS software generates 3-D topographic surfaces for acid-base equilibrium studies that portray pH and buffer capacity behavior during titration and dilution procedures. Topo surfaces are created by plotting computed pH and buffer capacity values above a composition grid with volume of NaOH as the x-axis and overall system dilution as the y-axis. What emerge are surface features that correspond to pH and buffer behaviors in aqueous solutions. Topo surfaces are created for pH, log buffer capacity and linear buffer capacity. Equivalence point breaks become pH cliffs and logarithmic buffer capacity canyons that grow shallower with dilution. Areas of high buffer capacity become rounded ridges. Dilution alone generates 45° ramps. Example systems include acetic acid, CH3COOH (a weak monoprotic acid); hydrochloric acid, HCl (a strong acid); oxalic acid, HOOCCOOH (a weak diprotic acid) and L-glutamic acid hydrochloride, C5H9NO4·HCl (a weak triprotic acid). The Supplementary files include a copy of the interactive BufCap TOPOS program as a downloadable Excel workbook. Its macro-enabled spreadsheets quickly generate surfaces for any mono-, di-, or triprotic acid. Only acid dissociation constants, Ka values, need be changed. Other materials include a PowerPoint lecture, materials/suggested laboratory activities for teaching with BufCap TOPOS, and derivation of new equations that permit the calculation of buffer capacities for titration/dilution composition grid points.
Publication Date
12-2020
Document Type
Book
Rights
© 2020 Garon C. Smith and Md Mainul Hossain
Creative Commons License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
Disciplines
Chemistry
Recommended Citation
Smith, Garon C. and Hossain, Md Mainul, "Chapter 1.2: Visualization of Buffer Capacity with 3-D Topos: Buffer Ridges, Equivalence Point Canyons and Dilution Ramps" (2020). Water Topos: A 3-D Trend Surface Approach to Viewing and Teaching Aqueous Equilibrium Chemistry. 3.
https://scholarworks.umt.edu/topos/3
Comments
This document is the Accepted Manuscript version of a Published Work that appeared in final form in the Journal of Chemical Education, copyright © American Chemical Society and the Division of Chemical Education, Inc., after peer review and technical editing by the publisher. Access the final edited and published work.